Domestic And Worldwide Shipping Of Biological Material And Experimental Medicines At A Controlled Temperature (-20°C; +2°C/+8°C; +15°C/+25°C)
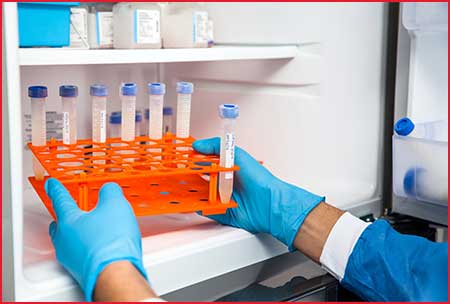
Transporting biological or diagnostic material requires specialized transport solutions. Biological or diagnostic material which comprises, for example, human tissue samples, organic liquids, blood samples, etc. must be preserved and transported refrigerated or in a frozen state. We have both the devices and the means to ensure the cold chain is managed correctly throughout the entire transport cycle for clinical trials.
ITAVIA has set up a dedicated division to centralize the operational management of clinical trials. The division’s operational staff are on call 24 hours day. Project activities are supervised and coordinated by ITAVIA staff members with years of experience in organization and operations and who interface and have key account functions with the clinical operation teams involved.

Speed and safety are essential for the management of clinical trials. ITAVIA satisfies these requirements with its dedicated transport solutions which ensure collection and delivery are carried out within the time frames laid down by the client, and with no intermediate stops along routes.
The state-of-the-art reusable packaging solutions for biological samples adopted by ITAVIA ensure a temperature range from -50°/+2°/+8°, +15°/+25° and maintain the product temperature for at least 96 hours. The packaging is suitable for both road transportation and air shipments and does not require any maintenance during shipping.
LE NOSTRE CERTIFICAZIONI





Licences
- Registration No. RMT5823577J in the Provincial Register for the Transport of Goods on behalf of Third Parties within Italy, in vehicles with a fully loaded overall weight of 1.5 tonnes
- Trucking transportation permit No. N GA3X8L for transport of goods on behalf of third parties in heavy duty vehicles issued by the M.C.T.C. of Rome on July 7 1986
- Registration No. 646 of 22/12/2009 in the interprovincial Roll of Freight Companies
- EU Licence No. 00072822 for international road transport of goods on behalf of third parties
- Compliance with local customs clearance procedures
- Permit No. 44513809 from the Italian Customs Agency for the utilization of the Customs Electronic Data Interchange system (EDI).